Blueprints of the Universe: How Atomic Models Evolved
Have you ever wondered how we all came to exist? Or what we are made up of? Well the
The answer to that is matter! Matter is anything that has mass or takes up space. But have you also thought about what matter is made of? And we will be answering that question in this article.
So, what is matter made up of? Matter is made up of atoms, these can be thought of as the basic building blocks of matter. Atoms are the smallest components of the world, and everything and everyone is made up of it. But within these atoms there are three very important sub-atomic particles known as protons which are positively charged, electrons which are negatively charged, and lastly, we have the neutron, and its charge is neutral meaning its neither positive nor negative. The atom is made up of two components: the central nucleus and the orbits/shells around this nucleus. And this is where the three sub-atomic particles come into play. Firstly the nucleus is made up of neutrons and protons which are in the center of the atom. Then there are the electrons which orbit in the ‘shells’ around the nucleus.
But how did we come to this conclusion? How did we know there was a nucleus and shells? And how did we know there were 3 sub-atomic particles? How did we even know atoms existed? Well, the atom’s structure evolved over time. And the journey of the atom began 2500 years ago.
It all started with a Greek man named Democritus, who was widely known for his wisdom and his first philosophy of the atom. At this point we can already tell this man was a philosopher, he proposed the idea of the atom in 430 BCE. He said that the universe was made up of small particles named ‘Atomos’. Which means ‘indivisible’ meaning nothing was smaller than these atoms. And he also said there were infinite of these and they were constantly in motion. But Democritus didn’t have a model of the atom, he thought they were invisible. Therefore he thought of it as ‘Nothing’.
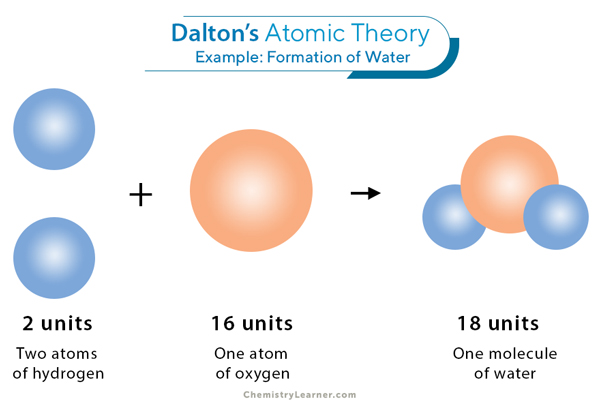
Secondly, we have John Dalton, he was an English chemist and physicist, who proposed his theory in 1803. And he proposed that each element in the periodic table has its own unique atom, which meant that every element had a different type of atom corresponding to that specific element. He also said that the atom was indivisible (He used Democritus’s theory) and indestructible with distinct masses and properties. He didn't physically see atoms but provided evidence for their existence through experiments with gases and observations of chemical reactions, particularly “The Law of Multiple Proportions”, which showed that elements combine in fixed whole-number ratios to form compounds. And his model was called “The Solid Sphere Model” or “The Bowling Ball Model” which looked like this: This was Dalton’s Theory:
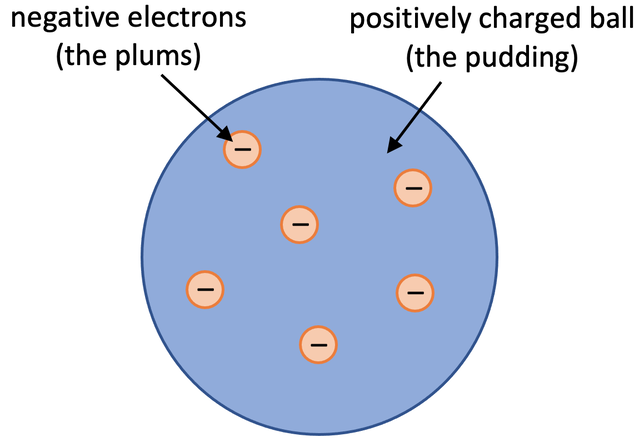
Thirdly, we have JJ Thomson and in 1897 he discovered the electron which is the negatively charged sub-atomic particle. He demonstrated that atoms were not indivisible but are made of smaller negatively charged particles. He conducted experiments with cathode rays to show these smaller sub-atomic particles, and they were much lighter than any other atom in the world. His experiment used cathode rays, a beam of light in a vacuum tube caused by high voltage. By observing the cathode rays deflect towards a positive electric plate and away from a negative one, Thomson concluded they consisted of negatively charged particles. Based on his discovery, Thomson proposed the plum pudding model, where negatively charged electrons are inside a sphere of positive charge. And his model is named after plum pudding because it looked like one, the electrons were the plums inside, and the pudding itself is the positively charged sphere. And this is how it looked:
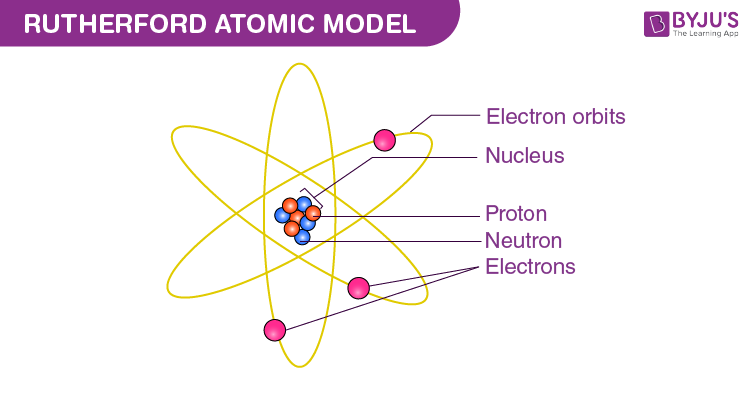
Fourthly, we have Ernest Rutherford which in 1911 discovered the central nucleus in the atom, he also discovered the proton, and he did this by using the gold foil experiment, he did this by shooting small/tiny alpha particles at a piece of gold foil most of it went straight through the foil, but at about 1/8000 chance the particle would scatter around rapidly, this showed him that there was a small, dense positively charged core in the center and he called it the nucleus, and because of this he also determined that 99.95% of the atom was just empty space. This also led to the discovery of the positively charged sub-atomic particle called the proton, which inhabited the nucleus. After the experiment he said this:
"It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you"
And this was his model known as “The Planetary Model”:
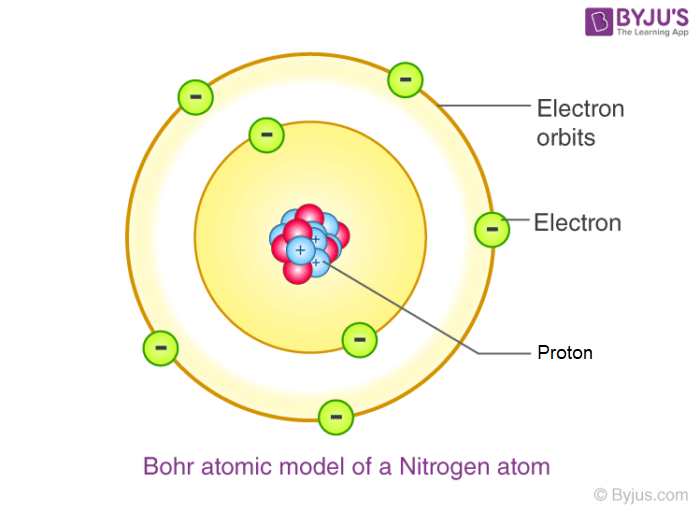
Fifthly, Niels Bohr, who introduced his atomic model by publishing three papers in 1913. This model, which became known as the Bohr atom, was a modification of Ernest Rutherford's planetary model, adding the concept that electrons orbit the nucleus in specific energy levels. And because of this he discovered that atoms had shells/orbits which led to the second to last model. His model was also called the planetary model. This is because it looked like the electrons were the planets and the nucleus was the sun, and that the electrons were orbiting the nucleus as it was the ‘Sun’.
After Niels Bohr came up with his model of the atom in 1913, scientists were excited. His idea of electrons moving around the nucleus in fixed orbits helped explain things like the colors given off by hydrogen gas. But soon, problems started to show up. Bohr’s model didn’t work well for atoms with more than one electron, and it couldn’t explain why electrons didn’t just crash into the nucleus.
In the 1920s, two scientists, Erwin Schrödinger and Werner Heisenberg, came up with new ideas that changed the atomic model again. Their work led to what we now call the Quantum Mechanical Model, or the Modern Atomic Model.
Instead of thinking of electrons as tiny planets orbiting the nucleus in neat circles (like Bohr did), Schrödinger said that electrons act more like waves. He created a very complicated equation, called the Schrödinger equation, which is used to figure out where an electron is most likely to be. This idea is all about probability. In simple words, we can’t say exactly where an electron is, but we can say where it’s probably going to be.
The area where an electron is likely to be found is called an orbital. These orbitals aren’t just circles, they have different shapes, like spheres, dumbbells, and even more complex forms. This model helped explain how electrons are arranged in atoms, especially for elements with lots of electrons.
At the same time, Werner Heisenberg came up with something called the Uncertainty Principle. He said that it’s impossible to know exactly where an electron is and how fast it’s moving at the same time. This might sound strange, but it’s actually a big idea in modern physics. It means that electrons don’t move in perfect paths like planets; instead, they sort of exist in a cloud around the nucleus.
This cloud-like picture of electrons is what we now believe is closest to the truth. The Quantum Mechanical Model doesn’t try to show electrons as little balls zooming in circles. Instead, it shows them as being somewhere in a fluffy/fuzzy area around the nucleus, based on probability.
Another big idea that came from this model is called electron spin. This means each electron has a tiny “twist,” kind of like how a top spins. This helps explain how electrons fill up orbitals, and why atoms behave the way they do in chemical reactions.
This model also helps us understand the Periodic Table better. It explains why elements in the same group similar properties have because their outer electrons are arranged in similar ways.
Thanks to Schrödinger and Heisenberg, the Quantum Mechanical Model became the new and improved version of the atom. It’s much more accurate than Bohr’s model and works for all elements, not just hydrogen. Scientists still use this model today to explain things in chemistry and physics, and it's the reason we understand things like how lasers work, how electronics are built, and even how stars burn.
In conclusion, after Bohr’s model, scientists realized that atoms are way more complicated than they first thought. With the work of Schrödinger and Heisenberg, the modern atomic model was born, a model based on probability, electron clouds, and energy levels. It changed how we understand matter and laid the foundation for much of modern science.
Article Written by Nishant Jothilingam
Comments ()